Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant, clonal plasma cell disorder, characterized by the presence of a monoclonal (M) protein in serum, <10% clonal plasma cells in the bone marrow, and absence of end-organ damage attributable to multiple myeloma (MM). MGUS incidence is estimated as 0.3% and 3% among those <50 years and >50 years, respectively. Although renal failure is a defining feature of MM, frequently patients (pts) with a variety of renal pathologies have less than 10% plasma cell burden in the bone marrow, therefore they cannot be categorized as MM to be eligible for an anti-myeloma therapy. However, it is clear that there is a causative relationship between MGUS and the pathologic process in kidneys in at least a subset patient and starting anti-myeloma therapy potentially can reverse or slow down the renal insult caused by MGUS. This led to the creation of the new entity and term, "monoclonal gammopathy of renal significance" (MGRS), to properly convey the nature of these relationship. Therefore, MGRS depicts a subset of MGUS and ascertains that the significance of the monoclonal gammopathy is no longer undetermined.
The epidemiology and health care burden of MGRS remains largely unknown. The incidence of MGRS, based on reports from single institutions, is estimated about 6%-10% of cases of MGUS (Nelson Leung et al. Blood. 2012), which suggests a total prevalence of 0.5-1% in the total United States population, accounting for a significant health care burden of 1.5-3 million cases with MGRS. Since pathologic characterization of MGRS is recent, a novel study to define the epidemiology of this subset of MGUS, its temporal course in relation to chronic kidney disease kidney (CKD), the risk factors for progression to end stage renal disease (ESRD) is urgently needed.
Methods: All pts from the University Hospitals Cleveland Medical Center, Cleveland, OH, diagnosed with CKD from 2000 to 2019 were included. We identified all cases with diagnosis of MGUS by ICD code. Time to end stage renal disease (TTESRD) was measured from the date of diagnosis of CKD to the date of diagnosis of ESRD and was censored at the date of last follow-up for those without ESRD with death as competing risk. The overall survival (OS) was measured from the date of diagnosis of CKD to the date of death The Fine and Gray method was used for comparisons of cumulative incidence of ESRD between groups. The effect of important factors on TTESRD was further evaluated using multivariable Fine and Gray method. Survivor distribution was estimated using Kaplan-Meier methods and the difference of OS between/among groups was examined by log-rank test.
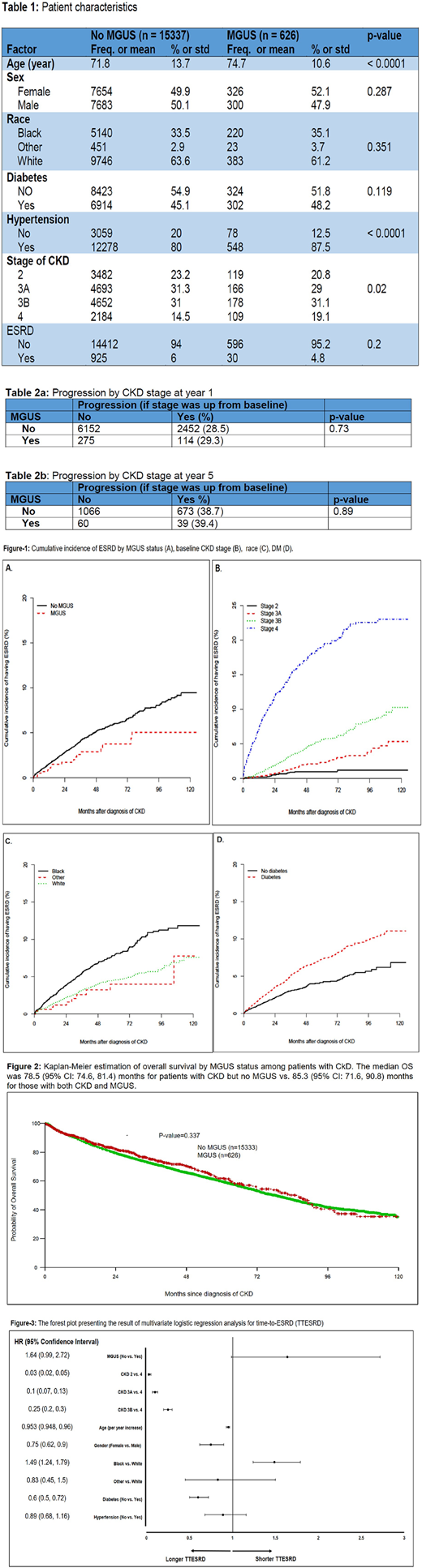
Results: The final data for the statistical analysis reported here contains 626 pts with MGUS and CKD and 15337 pts with CKD alone. The median follow up was 19.7 (range: 0.03, 128) months. Patient's characteristics and distribution of diabetes (DM), hypertension (HTN) and CKD stage at both cohorts are shown in Table-1.
Progression to ESRD: There was no difference between rate of progression to ESRD (as the binary outcome) in the cohort of pts with CKD and MGUS and the cohort of CKD without MGUS, 4.8% vs. 6%, respectively (p: 0.2). Univariate analysis of TTESRD showed lack of MGUS, Black race, DM, and CKD stage at baseline as statistically significant factors associated to progression to ESRD (Fig.1). Multivariable analysis on TTESRD including factors (MGUS status, age, gender, race, CKD stage, diabetes and hypertension) demonstrated higher CKD stage at baseline, younger age at time of CKD diagnosis, male gender and black race as the significant factors are associated with shorter TTESRD (Fig.3).
Progression by CKD stage advancement: The CKD stage at baseline was compared to the CKD stage at year 1 and year 5 during follow-up. The progression by stage was 28.5% at year 1 for pts without MGUS vs. 29.3% for those with MGUS (p = 0.73). The progression by stage was 38.7% at year 5 for pts without MGUS vs. 39.4 % for those with MGUS (p = 0.89).There was no difference between median OS for pts with CKD with or without MGUS (Fig-2)
Conclusion: Here we presented the data of around 16,000 CKD pts based on the MGUS diagnosis in 19 years time span and we could not detect any higher trend for CKD pts with MGUS to develop ESRD or move to higher CKD stage. These data can suggest MGRS forms a quite thin slice of the whole MGUS population. Assessing finding of this study in a larger national database is warranted.
Caimi:Amgen: Other: Advisory Board; Kite Pharma: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board, Research Funding; Celgene: Speakers Bureau; Verastem: Other: Advisory Board; Bayer: Other: Advisory Board. de Lima:Incyte: Other: Personal Fees, advisory board; BMS: Other: Personal Fees, advisory board; Kadmon: Other: Personal Fees, Advisory board; Pfizer: Other: Personal fees, advisory board, Research Funding; Celgene: Research Funding. Malek:Takeda: Other: Advisory board , Speakers Bureau; Sanofi: Other: Advisory board; Amgen: Honoraria; Medpacto: Research Funding; Clegene: Other: Advisory board , Speakers Bureau; Janssen: Other: Advisory board, Speakers Bureau; Bluespark: Research Funding; Cumberland: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal